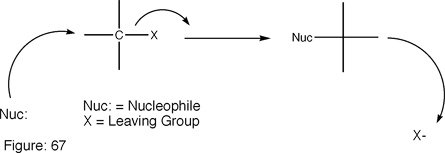

Sn2 reactions are very similar to Sn1 reactions. It is a type of nucleophilic substitution, but the main difference is the involvement of a leaving group and a rate determining step. An incoming group replaces a specific leaving group in a single step. (Note: this step makes up the rate limiting step of the Sn2 Reaction mechanism.)
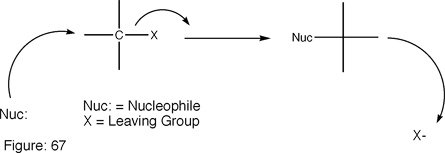
Above is the drawn Sn2 reaction. What happens here is the nucleophile comes in from the opposite side. The nucleophile forces the attached groups to shift their angles, in turn forcing the leaving group (usually a halogen) from its spot. A more detailed reaction is shown below.
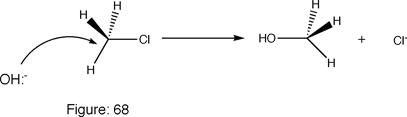
In this case, the hydroxyl group, the nucleophile, forces its way onto the carbon molecule's outermost valence electron level. This then forces the leaving group- the electrophile chlorine, off of the molecule.
A major difference between Sn1 and Sn2 is the mechanism behind the reaction. What happens in the Sn2 mechanism is the nucleophile is forcing the leaving group off in a single step, the rate determining step. Sn1 mechanisms use the rate determining step of converting carbon chain to a carbon chain with a charge-- see Sn1 Reactions on this site.
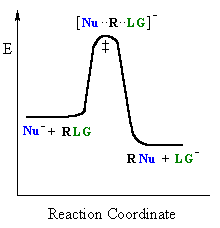
To be even more specific, Sn2 reactions have a transition state. The simultaneous formation of the carbon-nucleophile and the breaking of the carbon-leaving group bonds constitute the transition state. Thus, the central carbon has 5 groups attached to it at once.
The Sn2 mechanism depends on the solvent, temperature, concentration of the nucleophile and/or the leaving group.
All mechanisms of both Substitution and Elimination have a probability of producing products in both Substitution and Elimination. In other words each elimination reaction will produce substitution products- but what is produced of greater percentage determines what the mechanism is called. What determines what you get most out of a reaction relies of four major factors.
The most important factor: The type of halogenoalkane
| type of halogenoalkane | substitution or elimination? |
| primary | mainly substitution |
| secondary | both substitution and elimination |
| tertiary | mainly elimination |
For further explanation: a primary halogenoalkane will produce a majority of a substitution product, whereas a tertiary halogenoalkane will produce mainly an elimination product.
Other Factors Include:
The proportion of water to ethanol in the solvent: Water encourages substitution, Ethanol encourages elimination.
The temperature: Higher temperatures encourage elimination.
Concentration of the sodium or potassium hydroxide solution- Higher concentrations favour elimination.