

Sn1 Reactions are characterized by the substitution of an introduced nucleophile for a leaving group. What specifically characterizes an Sn1 reaction is the two step mechanism, where the leaving group is removed and creates an intermediate, and the nucleophile then comes in to create the new molecule.

Becuase the central carbon must have a charge before the nucleophile is capable of bonding, the period of removal of the leaving group is considered the rate-determining step. When the Intermediate Step is made, the nucleophile may attach itself determinate upon where and how it is attempting to attach (the faces of the carbocation). Although the nucleophile is capable of attaching itself to either face, the resulting molecule is usually the same.
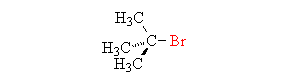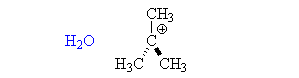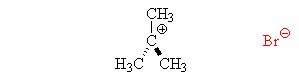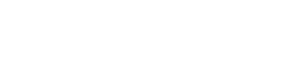
All mechanisms of both Substitution and Elimination have a probability of producing products in both Substitution and Elimination. In other words each elimination reaction will produce substitution products- but what is produced of greater percentage determines what the mechanism is called. What determines what you get most out of a reaction relies of four major factors.
The most important factor: The type of halogenoalkane
| type of halogenoalkane | substitution or elimination? |
| primary | mainly substitution |
| secondary | both substitution and elimination |
| tertiary | mainly elimination |
For further explanation: a primary halogenoalkane will produce a majority of a substitution product, whereas a tertiary halogenoalkane will produce mainly an elimination product.
Other Factors Include:
The proportion of water to ethanol in the solvent: Water encourages substitution, Ethanol encourages elimination.
The temperature: Higher temperatures encourage elimination.
Concentration of the sodium or potassium hydroxide solution- Higher concentrations favour elimination.