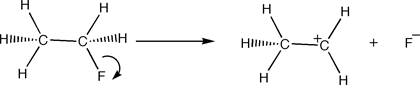

E1 reactions are elimination reactions with unimolecular kinetics. Because the reaction proceeds sequentially (the bonds break and form one after the other), E1 reactions require more activation energy than E2 reactions, in which the bonds are broken an formed simultaneously.
Step One: The leaving group departs and a carbocation is formed from the substrate.

Step Two: The carbocation loses a proton and forms the double bond.

All mechanisms of both Substitution and Elimination have a probability of producing products in both Substitution and Elimination. In other words, each elimination reaction will produce substitution products- but what is produced of greater percentage determines what the mechanism is called. What determines what you get most out of a reaction relies of four major factors.
The most important factor: The type of halogenoalkane
| type of halogenoalkane | substitution or elimination? |
| primary | mainly substitution |
| secondary | both substitution and elimination |
| tertiary | mainly elimination |
For further explanation: a primary halogenoalkane will produce a majority of a substitution product, whereas a tertiary halogenoalkane will produce mainly an elimination product.
Other Factors Include:
The proportion of water to ethanol in the solvent: Water encourages substitution, Ethanol encourages elimination.
The temperature: Higher temperatures encourage elimination.
Concentration of the sodium or potassium hydroxide solution- Higher concentrations favour elimination.