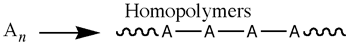
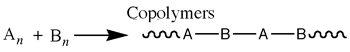

A polymer is a group of smaller units called monomers. A monomer is a group of molecules that can be attached in a repeating fashion in a reaction pathway called polymerization. (Note: A polymer can also be referred to as a macromolecule) There are two types of polymers: homopolymers and copolymer.
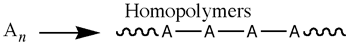
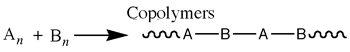
There are two types of polymerization reactions: chain-growth polymerization, and step-growth polymerizations. A chain-growth reaction begins when an initiator reacts with a monomer that generally has the same functional groups. The monomer then continues the reaction to form a polymer. A step-growth polymerization generally starts with a pool of monomers that have different functional groups. The different monomers then react with the other functional groups other than their own. The difference between a step-growth and a chain-growth is that step-growth reactions generally don't have a radical, cation, or anion at the end forcing the reaction.
Addition polymerization involves the linking together of molecules that contain double or triple bonds. These monomers, when placed together with each other, will break their pi bonds (double bonds or triple bonds) and seek out the electrons of a neighboring molecule. This results in a chain reaction that creates a large polymer, a repeating chain. Some examples of addition polymers are:

The free radical, or the attacking group in the reaction, (e.g. the Hydroxyl group) forces the ethene group to break its double bond, thus resulting in a group with an extra electron. Thus, this compound is then able to attack another ethene group, in the reaction shown below.
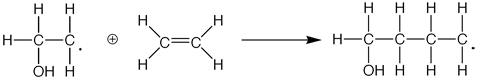
Condensation polymerization occurs when two monomers bond together by creating and then releasing a water molecule. As with addition polymerization, these reactions can continue until all of either substance runs out. The example shown below is a form of nylon, perhaps the most familiar polymer formed from third type of reaction. (Note: It is much easier to learn with actual examples)
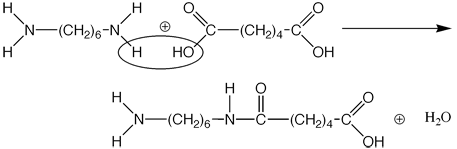
In this reaction, the hydrogen from the first compound, and the hydroxyl group of the second react, forming a water molecule, and forcing a C-N bond upon the larger molecule. This larger molecule, called a dimer (two monomers joined together, can further react because it has a reactive hydrogen and hydorxyl group still in place. Thus, we will write this reaction in a common notation for polymers, which you are almost sure to see sometime in your chemical career:
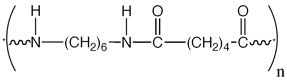
As you can see, the chemical placed in parenthesis is the offending repeating dimer. "n" represents the fact that there may be no end to the condensation process.