
E2 reactions require a strong base that accepts a proton at the same time that
the leaving group leaves. When the leaving group has left a transition
state is formed which bridges the two steps. E2 reactions have a lower
activation energy requirement than E1 reactions and tend to occur spontaneously.
The product of an E2, or elimination bimolecular reaction, is always one degree
more unsaturated than the reactants. For example an alkane reacting with a strong
base would produce an alkene. E2 reactions are second order, that is they require
two molecules to come together in order to proceed to completion. The strong base is
required in order to increase the reactions ability to occur.
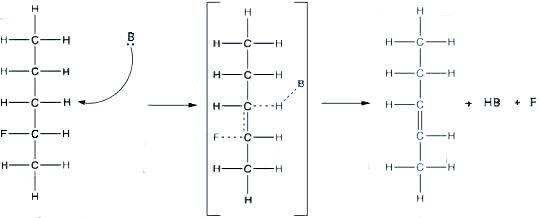
The reactants in an E2 reaction goes through a transition state in which the halogen and
the leaving group are breaking away and the double bond is being formed between the carbons.
Example of an E2 Reaction with a transistion state

All mechanisms of both Substitution and Elimination have a probability of producing products in both Substitution and Elimination. In other words each elimination reaction will produce substitution products- but what is produced of greater percentage determines what the mechanism is called. What determines what you get most out of a reaction relies of four major factors.
The most important factor: The type of halogenoalkane
| type of halogenoalkane | substitution or elimination? |
| primary | mainly substitution |
| secondary | both substitution and elimination |
| tertiary | mainly elimination |
For further explanation: a primary halogenoalkane will produce a majority of a substitution product, whereas a tertiary halogenoalkane will produce mainly an elimination product.
Other Factors Include:
The proportion of water to ethanol in the solvent: Water encourages substitution, Ethanol encourages elimination.
The temperature: Higher temperatures encourage elimination.
Concentration of the sodium or potassium hydroxide solution- Higher concentrations favour elimination.