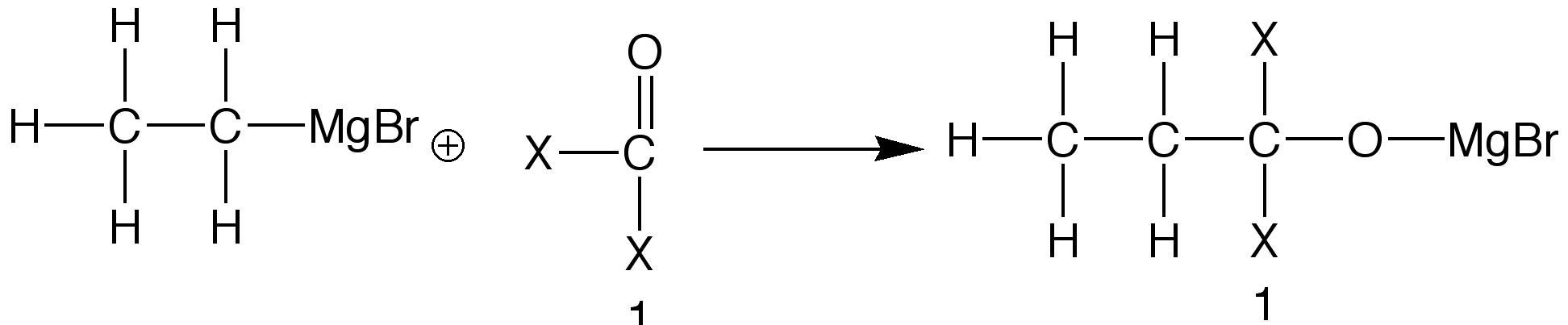

A Grignard reagent is an organic magnesium halide which is highly reactive when placed with any electrophilic group, such as aldehydes and ketones. These generally carry the general formula of RMgX, where R is the attached alkyl or aryl group, and X is the attached halogen. For the sake of simplicity (and also because I'm lazy), the example reactions shown here will contain alkyl groups.
Much of the exact mechanism of these reactions is still questionable, so we will focus on known and common reactions involving Grignard reactions:
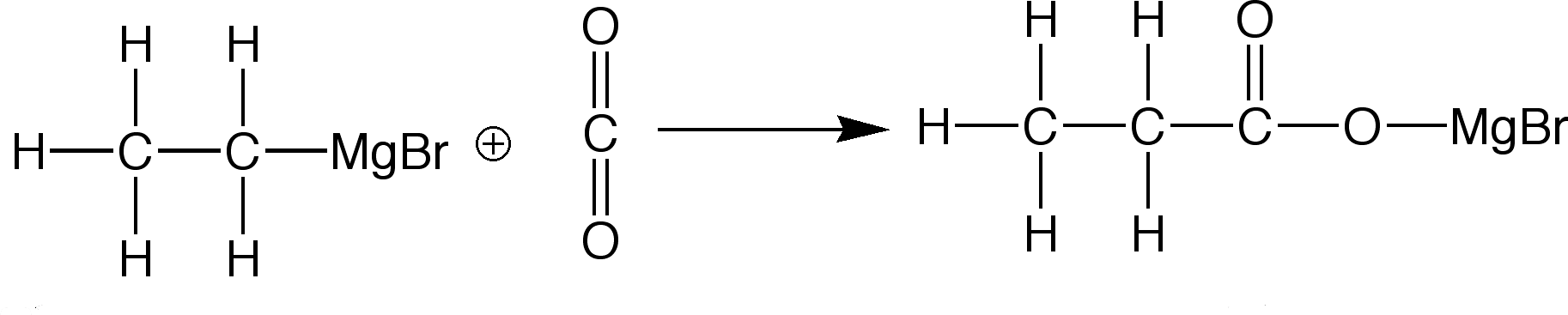
Reaction between Grignard Reagents and Carbon Dioxide:

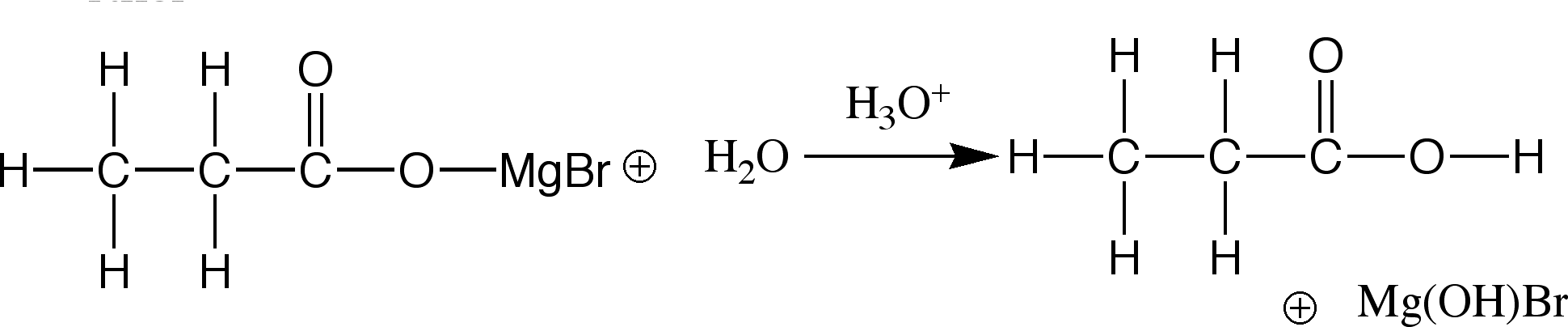
The product is then hydrolysed(added to water) in a dilute sulfuric acid to further the reaction
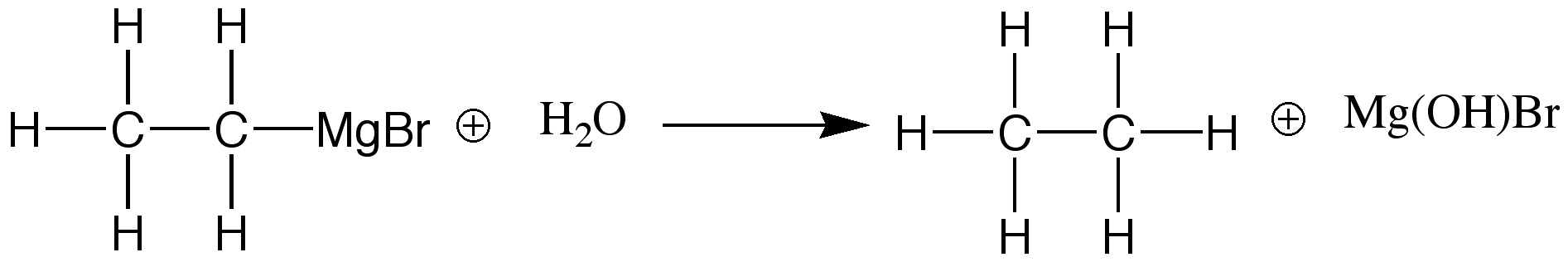
Reaction between Grignard Reagents and Water:
The next major group of examples involve carbonyl groups, or groups that contain a carbon-oxygen double bond, most commonly ALDEHYDES and KETONES. Though this particular mechanism may look difficult, almost all of these reactions proceed in the same manner. The mechanism merely breaks the Carbon-Oxygen double bond. The general aldehyde reaction is shown below, where X and X' can be any alkyl group or hydrogen:
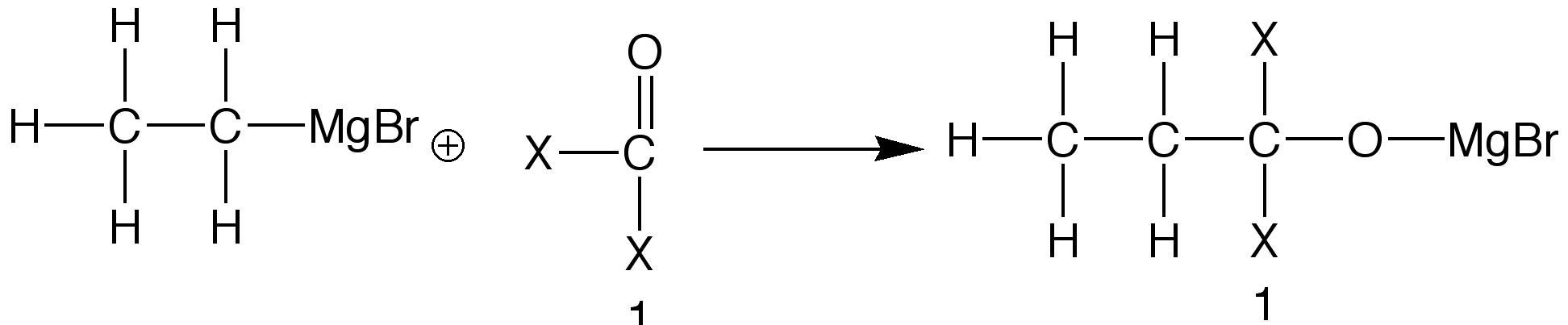
After this initial reaction, the product is usually hydrolysed, forming an alcohol.
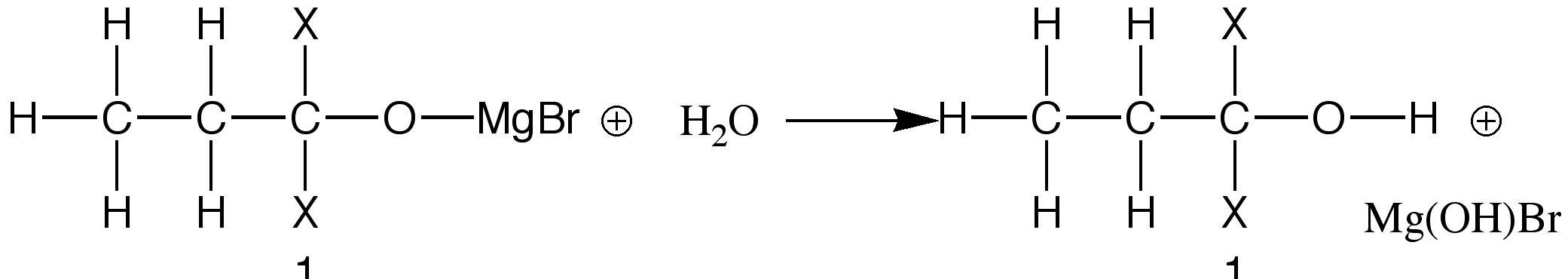
The alcohol obtained from the reaction depends on the groups attached to the original carbonyl compound. The formation of these alcohols is the primary use of Grignard Reagents within the scientific community.
The general formula for the Ketone reaction is exactly the same.