

This is an introduction to Geometric Isomerism, also know as Cis- Trans Isomerism. If you think you're in the wrong place or are unprepared for the horrors that lie ahead, then RUN! RUN AS FAST AS YOUR LEGS WILL CARRY YOU!
Now that we've weeded out the weaker and spindlier of you aspiring chemists, we'll get down to business with a simple definition of Geometric Isomerism:
Because these compounds are not able to rotate, two different forms of the same
compound can be formed. A cis- compound, where the offending molecules are placed on
the same side, and a trans- compound, where said molecules are placed on opposing sides.

cis-1,2- dibromoethene; trans-1,2-dibromoethene
As you can see from the example above, these are versions of the EXACT same compound, with the only
exception being the placement of the bromine.
Of course, this cis-trans type of isomerism can only happen if there are two DIFFERENT types of
molecules or alkyl groups on the left-hand carbon, and two different types of molecules or
alkyl groups on the right hand carbon. However, if all four groups are different, as is the case
below, cis and trans lose all their meaning.
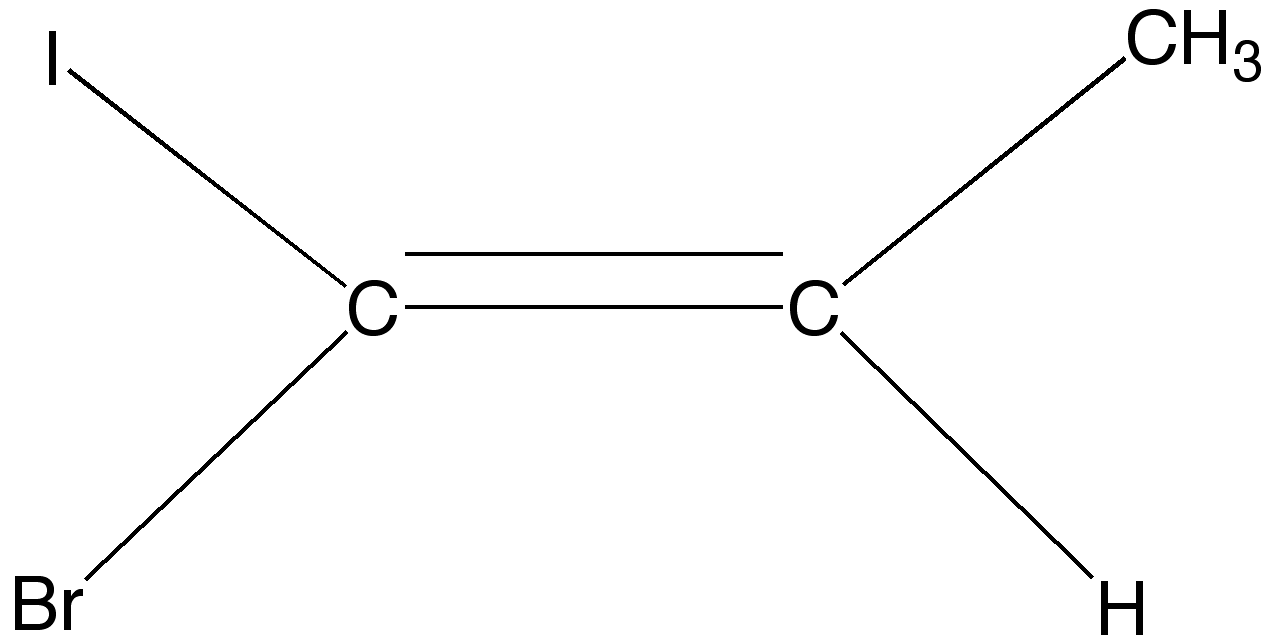
But, both fortunately and unfortunately for you,
these compounds will not be covered in this site.
Now you may be asking yourself, "Why is this important to the real world? Does it even make a difference if some molecules are switched around?" The answer, unfortunately, is yes. The differences between the cis and trans formations have the same compound have many physical differences between them, including but not limited to density, melting points, and boiling points.
Optical isomerism is a phenomenon in which two molecules have the same chemical formulae,
however, the individual molecules are arranged in such a way that the images of both molecules cannot be
superimposed. This causes the molecules to rotate a plane of light either clockwise or counter-clockwise.
Molecules that exhibit this behavior are called chiral (ky-ral) the different forms are called enantiomers.
Optical isomerism belongs to stereo isomerism along with geometrical isomerism.
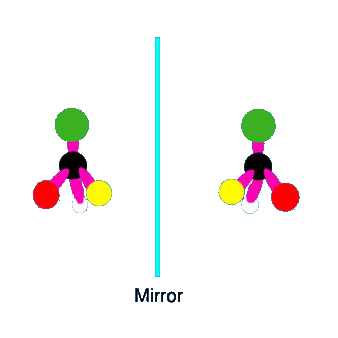
Structural isomerism: The same molecular formula of a structure but a different structural formula.
Structural Isomerism of Alkanes:
Example of Structural Isomerism
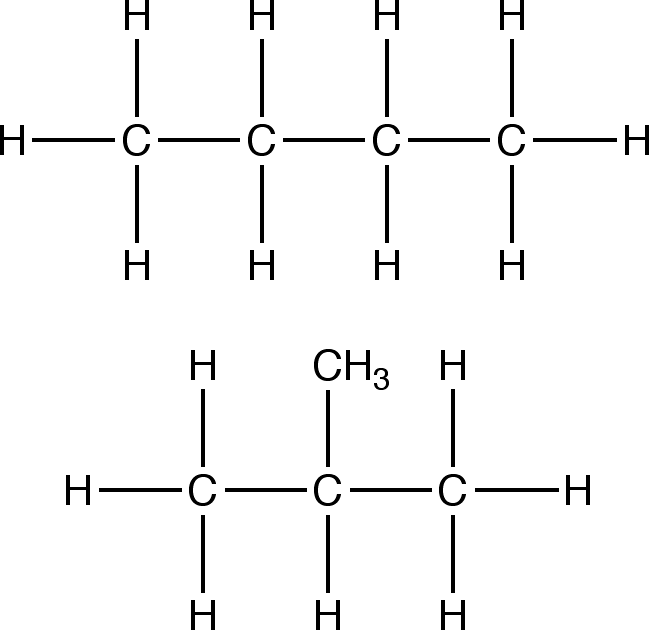
The isomerism of a molecule represents completely different organic compounds: not only are their atoms bound differently but they also have different chemical properties. An example in the change of physical properties is the difference in boiling point due to the molecular bonding of the isomers. For example, CH3CH2CH2CH3 boiling point is -0.6°C and the boiling point of CH(CH3) is 10.2°C. Same chemical formula, but different boiling points.