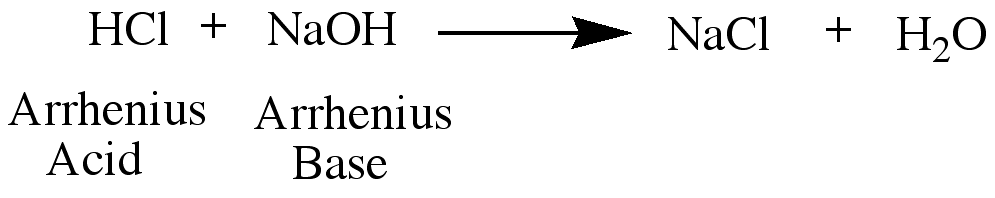

An arrhenius acid is a hydrogen donor, and an arrhenius base is a hydrogen acceptor. In other words,
An arrhenius acid is a source of H+ ions, and an arrhenius base is a source of OH- ions.
When a stable, soluble ionic compound is put into water, it disassociates into its component
ions. (This happens in an equilibrium reaction in which H2O strips the acid of a hydrogen ion, forming H3O+
ions. The H3O+ ions are commonly just referred to as H+.)

Brønstead Lowery acids & bases are proton donors, and proton receivers respectively. In other words,
A Brønstead Lowery acid gives an electron deficient H+ ion, and Brønstead Lowery base
accepts an electron deficient H+ ion.
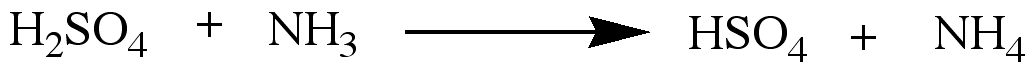
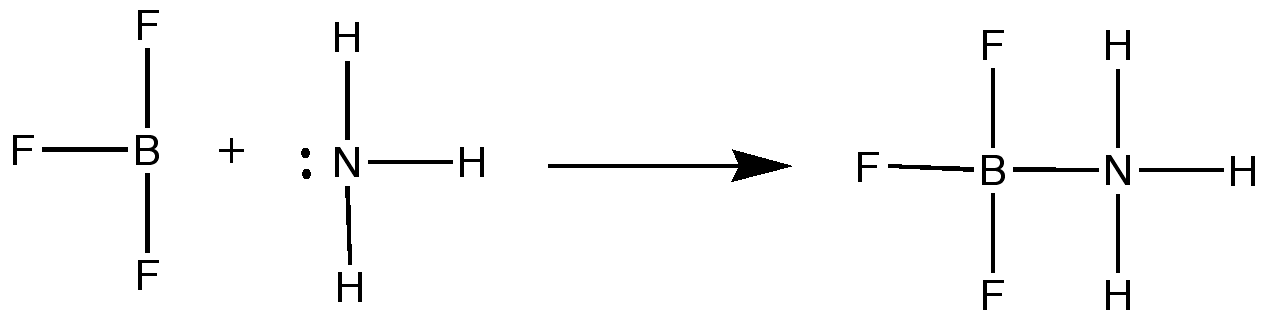
Lewis acids and bases are defined much broader than arrhenius, or Brønstead-Lowery. Lewis defined an acid
as a molecule that accepts an electron pair to form a covalent bond, and a base molecule donates an electron pair to form
a covalent bond. In other words, A Lewis acid is an electron pair acceptor, and a Lewis base is an electron pair donor.
For this reason, chemists call Lewis acids Electrophiles and Lewis bases Nucleophiles. In other words, a Lewis
acid is a "lover of electrons", and a Lewis base is a "lover of nuclei".
The strength of an acid is determined by how much it autoionizes compared to water.
Kinetics determines the strength of an acid through concentrations. In other words,
the strength of an acid or pH is determined by comparing the [H3O+] and
[A-] to the [HA] and [H20].